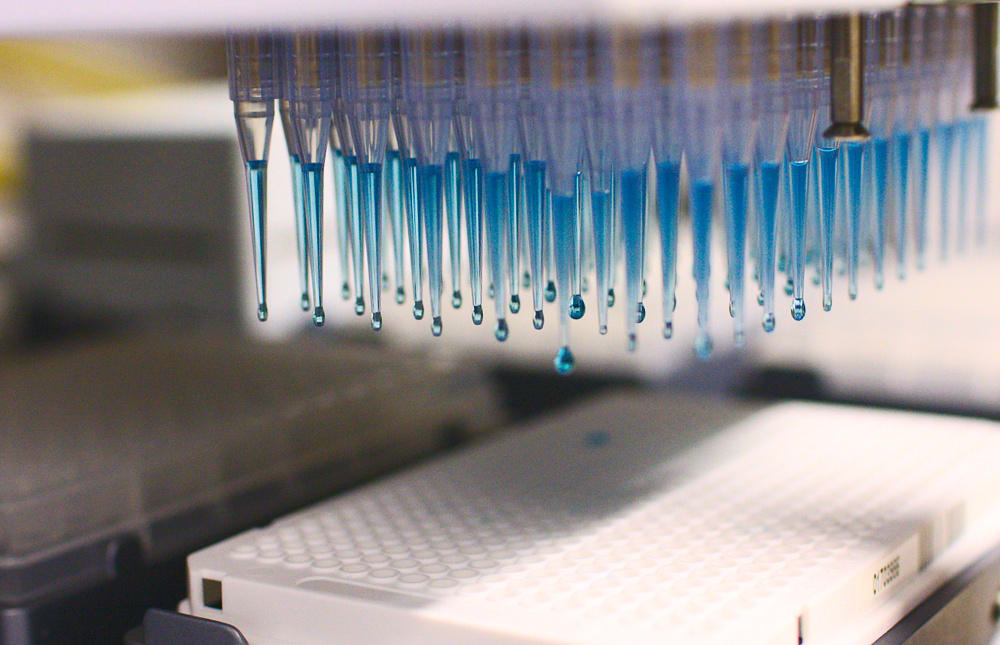
It is an automated drug discovery process that helps to find active compounds or leads quickly. A large number of compounds can be screened and different tests like chemical, genetic, or pharmacological can be performed by use of robots and software control.
The samples that show some positive results are referred to as hits and then assayed further to confirm and redefine the finding.
The main feature of HTS is its automation with robotics which allows mixing, incubation, and detection. About 100,000 compounds are screened in a single day. There are HTS robots that screen more than 100,000 compounds per day, and this is referred to as ultra-high throughput screening. Quality control is maintained both experimentally as well as with a computational approach which involves good plate design, selection of chemical controls, and statistical knowledge to measure the degree of differentiation to exclude inferior data.
HTS has certain limitations we cannot find out the bioavailability, pharmacokinetics, toxicity, and absolute specificity of the lead compound identified through it. It provides information about potency and activity. HTS has provided drugs for many diseases as well as for their prevention for example Cyclosporin A, Mevastatin, etc.
It is a method of experiment for scientists used in the discovery of the drug, moreover, it is an interlinking bond between biology and chemistry. This technique uses artificial intelligence, robots, and computer software which allows us to conduct many pharmacological tests at a very small pace of time and accurately. This process can help to identify an active compound, gene, and protein.
History:
It has its origin in natural products in 1986, by replacing synthetic compound of dimethyl sulfoxide with fermentation broths by decreasing assay volume up to 100 microlitres and 96 well plates. In 1989, the process reached a constant state by producing 7200 substances each week.
For almost 40% discovery portfolio HTS gave rise to “hits” by the year 1992. In 1995, it included the ADME target and by the year 1996, almost 90 molecules were tested for protein binding, stability parameters, etc. Similarly, the mutagenic assay was developed and in 1999 ADME HTS completely came under the discovery cycle.
Advantage:
- To screen many kinds of active compounds such as natural products, and combinatorial libraries.
- To screen array-like DNA, RNA, and protein chips.
- High speed of the assay
- Highly sensitive processes.
- Reproducible results.
- Effective to determine the ADME part of any drug or molecule.
Disadvantage:
- High cost
- Low quality
- Contamination chances are much less
- Require pure product
- Require the attention and safety of the product.